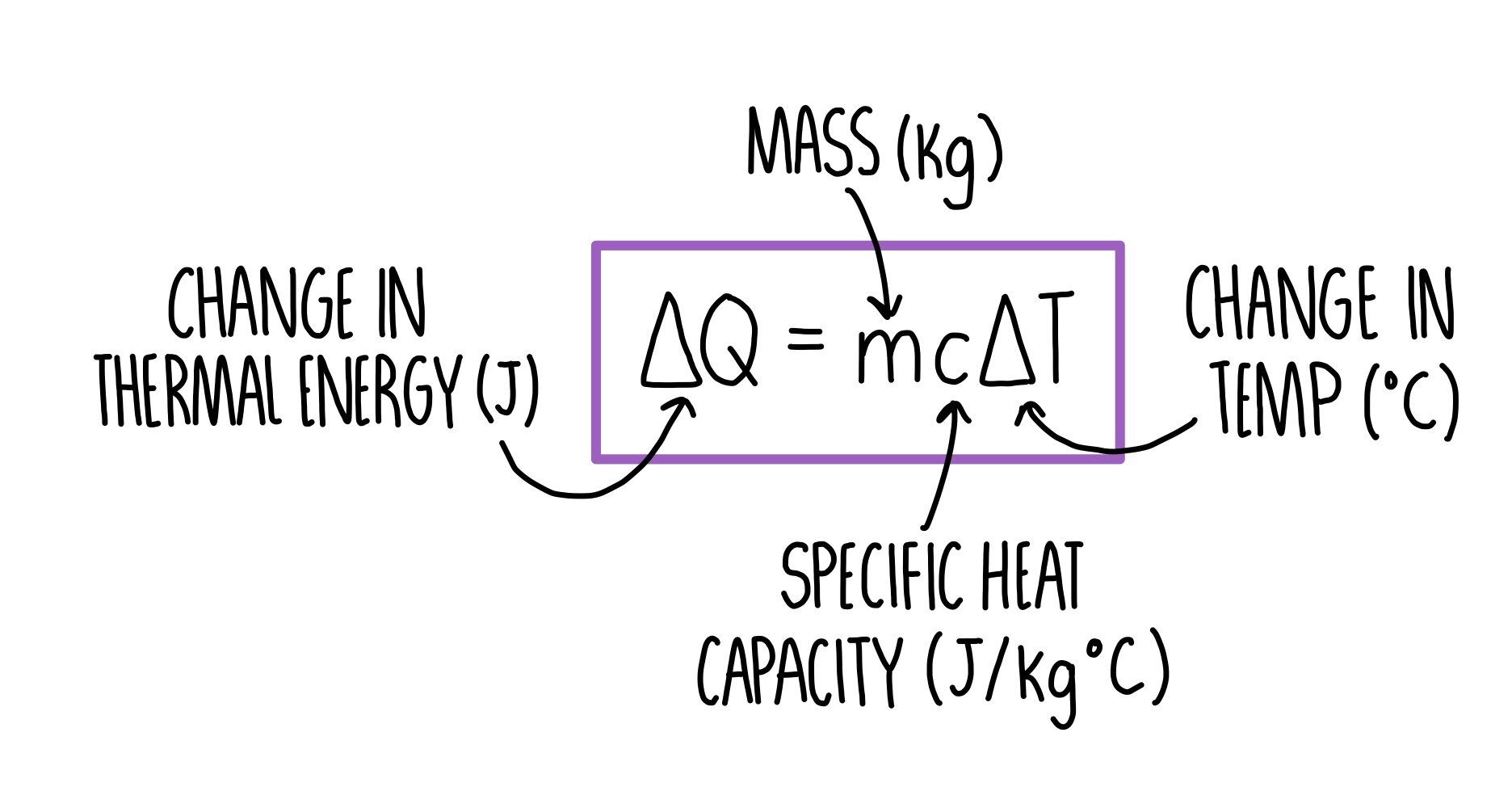
We have done two lab reports that discuss different topics The first one discussed the effect of temperature on average kinetic energy and average speed and what we concluded was that temperature was directly proportional to both average kinetic energy and average speed. Temperature is the average kinetic energy and as speed increases the average kinetic energy will increase, increasing the temperature. Our conclusion was Both particles would have the same graph shapes, but the difference is the numbers that came from the average speed, temperature, and average kinetic energy. The mass makes the set of smaller balls move faster which shows us that
mass is the factor that can change the speed and temperature of a substance



Comments
Post a Comment